If we start with 8000 atoms of radium-226, we embark on a captivating journey into the realm of radioactive decay and its multifaceted applications. This element, with its intriguing properties and historical significance, unravels a narrative that intertwines scientific principles with practical implications.
Radium-226, a naturally occurring radioactive isotope, stands as a testament to the power of nuclear reactions. Its decay chain, a cascade of transformations, releases energy and forms new elements, offering insights into the fundamental processes that shape our universe.
Radioactive Decay of Radium-226
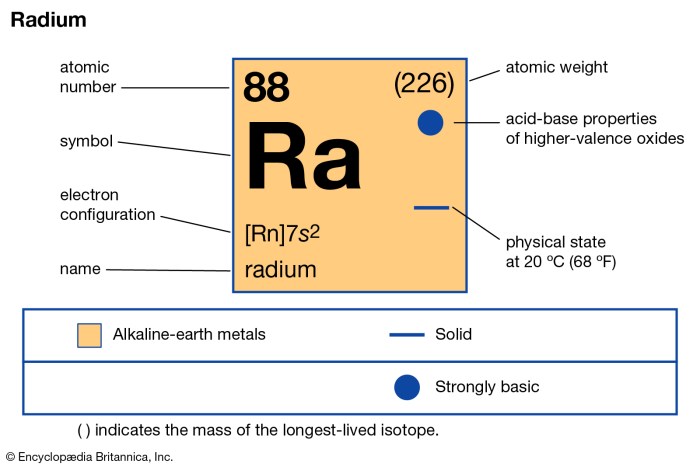
Radium-226 is a radioactive isotope with an atomic number of 88 and a mass number of 226. It is part of the uranium decay series and is the parent isotope of radon-222. Radium-226 undergoes radioactive decay through a series of alpha and beta emissions, ultimately forming the stable isotope lead-206.
Decay Chain of Radium-226, If we start with 8000 atoms of radium-226
The decay chain of radium-226 is as follows:
- Radium-226 (α, 1600 years) → Radon-222 (α, 3.82 days)
- Radon-222 (α, 3.82 days) → Polonium-218 (α, 3.05 minutes)
- Polonium-218 (β-, 3.05 minutes) → Lead-214 (β-, 26.8 minutes)
- Lead-214 (β-, 26.8 minutes) → Bismuth-214 (β-, 19.9 minutes)
- Bismuth-214 (β-, 19.9 minutes) → Polonium-214 (α, 164 microseconds)
- Polonium-214 (α, 164 microseconds) → Lead-210 (β-, 22.3 years)
- Lead-210 (β-, 22.3 years) → Bismuth-210 (β-, 5.01 days)
- Bismuth-210 (β-, 5.01 days) → Polonium-210 (α, 138.4 days)
- Polonium-210 (α, 138.4 days) → Lead-206 (stable)
Half-Life and Decay Rate: If We Start With 8000 Atoms Of Radium-226
The half-life of a radioactive isotope is the amount of time it takes for half of the atoms in a sample to decay. The half-life of radium-226 is 1600 years. This means that after 1600 years, half of the atoms in a sample of radium-226 will have decayed.
The decay rate of a radioactive isotope is the number of atoms that decay per unit time. The decay rate of radium-226 is given by the following equation:
λ = ln(2) / t 1/2
where λ is the decay rate, and t 1/2is the half-life.
For radium-226, the decay rate is:
λ = ln(2) / 1600 years = 4.33 × 10 -4years -1
Applications of Radium-226

Radium-226 has a number of applications, including:
- Medicine:Radium-226 is used in radiation therapy to treat cancer. It is also used in the production of radon gas, which is used in radon therapy for the treatment of chronic pain.
- Industry:Radium-226 is used in the production of self-luminous devices, such as watch dials and aircraft instrument panels. It is also used as a neutron source in nuclear reactors.
- Research:Radium-226 is used in a variety of research applications, including the study of radioactive decay and the development of new nuclear technologies.
Health and Safety Considerations
Radium-226 is a radioactive substance and can be harmful to human health. Exposure to radium-226 can cause a variety of health problems, including cancer, birth defects, and developmental disorders.
The following precautions should be taken when handling or working with radium-226:
- Wear protective clothing, including gloves, a lab coat, and a respirator.
- Work in a well-ventilated area.
- Avoid contact with radium-226.
- Store radium-226 in a secure location.
- Dispose of radium-226 properly.
Comparison to Other Radioactive Isotopes
Radium-226 is one of a number of radioactive isotopes that are used in a variety of applications. Other radioactive isotopes include uranium-238, thorium-232, and plutonium-239.
The following table compares the properties of radium-226 with those of uranium-238 and thorium-232:
| Isotope | Half-Life | Decay Mode | Applications |
|---|---|---|---|
| Radium-226 | 1600 years | α and β | Medicine, industry, research |
| Uranium-238 | 4.47 billion years | α | Nuclear power, nuclear weapons |
| Thorium-232 | 14.05 billion years | α | Nuclear power, nuclear weapons |
Commonly Asked Questions
What is the half-life of radium-226?
1602 years
What are the historical applications of radium-226?
Radium-226 has been used in luminous paints, medical treatments, and neutron sources.
What are the potential health risks associated with radium-226?
Exposure to radium-226 can increase the risk of cancer and other health problems.